Abstract
Background: Although there has been population-based evidence on real-world patterns of BTKi use and early discontinuation in CLL, the experience in MCL has not been well described on a large scale. A single center study (Cheah et al, Ann Onc 2015) found many patients discontinued BTKi shortly after the initiation due to progression or intolerance; survival outcomes following discontinuation were very poor. These issues might be more challenging in the older population, given the prevalent comorbidities and frailty, and relatively limited treatment options for MCL compared to the younger group. In this population-based analysis, we used the SEER-Medicare database to examine real-world BTKi use and outcomes following discontinuation of BTKi in older patients with MCL.
Methods: We selected adults ≥ 66 years old, diagnosed with MCL 2007-2017, with continuous Medicare A/B/D coverage (through 2019 or death), who received MCL therapy. We captured the regimens of 1st through 5th lines based on the outpatient claims and limited our analysis to patients who received their first BTKi (ibrutinib or acalabrutinib as a single agent or combined with other chemoimmunotherapy) during 2nd to 4th line of therapy. We described the baseline characteristics of the overall study population. We reported the median duration of BTKi use with interquartile range (IQR) and the rates of continuation of the same BTKi at 12 and 24 months (m) by applying Kaplan-Meier (K-M) method (discontinuation or death as complementary events of interest). We estimated compliance in patients who received ≥ 2 consecutive BTKi prescriptions, based on proportion of days covered (PDC), and defined good compliance as PDC ≥ 80%. We used K-M method to estimate overall survival (OS) in the overall population. Similarly, we estimated OS following the initiation of next line therapy (non-BTKi) following BTKi discontinuation and described the regimens used following BTKi.
Results: We included 334 patients (median age: 74, 67% men, 95% White, 14% with Elixhauser comorbidity score ≥ 3, 19% with frailty), with 66%, 25%, and 9% receiving BTKi at the 2nd-4th line, respectively. The median follow-up was 37.3 m since the initiation of BTKi and 78.1 m since the start of first line therapy. The median duration of BTKi therapy was 4.6 m (IQR: 2.1-13.0 m). Among patients alive at 12 and 24 m after initiation of BTKi, 42% and 27% had continued BTKi, respectively. Among patients with at least two consecutive BTKi prescriptions (N=284), 88% met the criteria of good compliance. The OS since initiation of BTKi was 19.3 m.
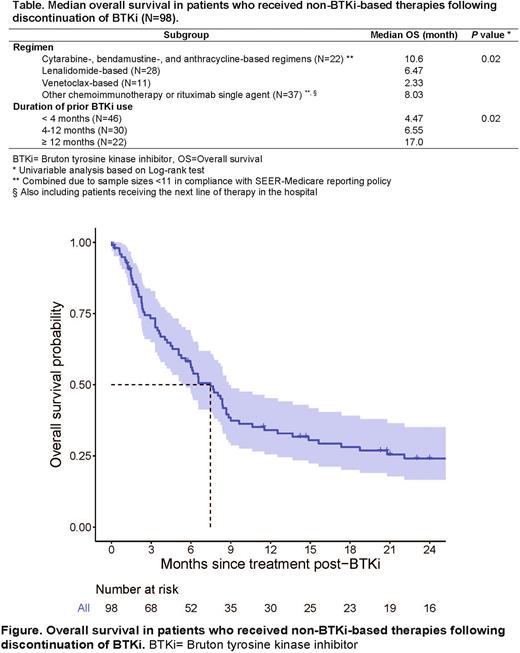
During follow-up, 188 (56%) patients discontinued their first BTKi agents and 73 patients died while being on BTKi. Following discontinuation, 132 patients received a next line of therapy, 98 received non-BTKi-based regimens (see Table for details of regimens) and the rest switched to another BTKi agent. During a median follow-up of 22.2 m, the median OS was 7.5 m following the initiation of non-BTKi-based next line of therapies (Figure). The OS was poor regardless the regimens of next line of therapy following BTKi. Patients who received BTKi for ≥ 1 year had significantly better OS (Table).
Conclusions: As one of the first population-based real-world analysis on BTKi discontinuation among MCL patients, our study demonstrated that the duration of BTKi use (4.6 months) in older patients with MCL is much shorter than that reported in the clinical trial setting (8.3 months; Wang et al, NEJM 2013). Outcomes after the discontinuation of BTKi remained dismal even with other novel therapies as the next line of treatment, e.g., lenalidomide- or venetoclax-based regimens. Improvements to both real-world administration of BTKi and novel combinations are likely needed to improve real-world outcomes in older patients with MCL.
Disclosures
Kothari:Incyte Pharmaceuticals: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Radyus Research: Consultancy, Honoraria. Zeidan:Astex, Medimmune, Astrazeneca, ADC Therapeutics: Research Funding; Gilead, Kura, Loxo Oncology: Consultancy, Honoraria, Other: Clinical Trial Committee; Pfizer, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, Amgen, Aprea, Gilead, Kura, Loxo Oncology, Otsuka, Jazz, Agios, Acceleron, Astellas, Daiichi-Sankyo, Cardinal Health, Taiho, Seattle Genetics, BeyondSpring, Ionis, Epizyme, Janssen, Syndax, Genentec: Consultancy, Honoraria, Other: Advisory Boards; Celgene/BMS, Novartis, Cardiff Oncology, AbbVie, Pfizer, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, Amgen, Aprea, Astex, Pfizer, Medimmune/AstraZeneca, ADC Therapeutics: Research Funding; Celgene/BMS, AbbVie, Pfizer, Boeringer-Ingelheim, Trovagene, Cardiff Oncology, Incyte, Takeda, Novartis, Aprea, Amgen, Otsuka: Consultancy, Honoraria, Research Funding; Novartis, Cardiff Oncology, Pfizer: Other: Travel Support; Celgene/BMS, Novartis, Cardiff Oncology, AbbVie: Consultancy, Honoraria, Other: Advisory Board; Celgene/BMS, Novartis, AbbVie, Gilead, Kura, Loxo Oncology, Geron: Other: Clinical Trial Committee; Jazz, Agios, Acceleron, Astellas, Daiichi Sankyo, Cardinal Health, Taiho, Seattle Genetics, Beyondspring, Gilead, Kura, Tyme, Janssen, Syndax, Geron, Ionis, Epizyme: Consultancy, Honoraria. Podoltsev:Bristol-Myers Squibb: Honoraria; Incyte: Honoraria; Celgene: Honoraria; Cogent Biosciences: Other: Independent Data Review Committee ; Pfizer: Honoraria; CTI BioPharma: Honoraria; PharmaEssentia: Honoraria; AbbVie: Honoraria; Blueprint Medicines: Honoraria; Novartis: Honoraria; Agios Pharmaceuticals: Honoraria; Constellation Pharmaceuticals: Honoraria. Shallis:Bristol Myers Squibb and Gilead Sciences, Inc: Honoraria; Gilead Sciences, Inc.: Honoraria. Wang:Celgene: Research Funding. Ma:Bristol Myers Squibb: Consultancy. Huntington:Tyme: Consultancy; Seattle Genetics: Consultancy, Honoraria; Merck: Consultancy; Epizyme: Consultancy, Honoraria; ADC Therapeutics: Consultancy, Honoraria; AstraZeneca: Consultancy; Beigene: Consultancy; FlatonIron Health: Consultancy; AbbVie: Consultancy; Genentech: Consultancy; Janssen: Consultancy; Agios: Research Funding; Debiopharm Group: Research Funding; TG Therapeutics: Consultancy, Research Funding; DTRM Biopharm: Research Funding; Pharmacyclics: Honoraria; AstraZeneca: Consultancy; Arvinas, Novartis, Servier, Bayer, SeaGen: Consultancy; Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal